The Asymmetric World: A Nobel Tale
Review
Subhajit Chakraborty

Tweet
This beautiful world and Mother Nature host an alluring interplay of different symmetric and asymmetric objects on the basis of which all life processes, climatic phenomena, and other scientific activities occur. Scientists have been trying to decode this interplay of symmetry and asymmetry for a long, long time now, and it seems there is significant growth in this process.
The chemists were also not an exception; in this case, they had ventured deep into the world where they wanted to define every molecule on the basis of symmetry. This led to the foundation of some of the most significant symmetry-related theories, which further led to the foundation of modern organic chemistry. These concepts have been central to modern chemistry for a really long time now, and the importance of this remark was reflected when the 2021 Nobel Prize Chemistry was announced. How is that related to the Nobel prize? Well, fasten your seatbelts, and let us dive into the land of asymmetries and some spicy organic chemistry to try to find our answer.
This year, the Nobel Prize in Chemistry was awarded to Prof. Benjamin List of Cologne University and Prof. David Macmillan of Princeton University for their brilliant contribution in “Asymmetric Organocatalysis”.
Now, these terms seem pretty complex, so let us first try to understand the primary context of the term Asymmetric Synthesis.
Asymmetric synthesis refers to the preferential synthesis of only one product in a reaction where more than one compound is forming. It’s like choosing between two items from a mixture.
Now that we are somewhat familiar with asymmetric reactions, then comes the fair question, "What about the catalysts?"
Let us first understand some basic properties of catalysts. Catalysts are a group of chemical compounds which are involved in fastening chemical reactions and improving the quality of reactions. These chemicals themselves remain intact throughout the course of the reaction.
The question regarding these catalysts has led to the foundation of asymmetric catalysis. The majority of reactions in organic chemistry employ either metal as catalysts or some enzymes for catalysis. These catalysts are highly effective but there are some problems and issues with them.
Let us talk about the metal catalysts first:
Now, let us look into enzyme catalysis and try to understand the problems related to these types of reactions. These reactions occur readily in the body of living organisms in a pretty good fashion but there are cases where chemists try to mimic these reactions in the lab. But practically speaking, it would take a lifetime to just synthesise one enzyme molecule, as these molecules are gigantic with various structural complexities. So, it may not be feasible to recreate these enzyme-catalyzed reactions in laboratory conditions. This is one of the limitations of using enzymes as catalysts.
So, these drawbacks of the catalysts indicated a new discovery, and a brilliant question took birth in the minds of Prof. Benjamin List and Prof. David MacMillan. It was, “What if organic compounds can catalyze reactions?” or, more specifically, catalyze these asymmetric reactions. They immediately started their independent journeys towards a common goal: to find organic asymmetric catalysts. Hence, “asymmetric organocatalysis” started its beautiful voyage.
Let us try to understand the independent work that led them to a similar yet very powerful conclusion.
Prof. List worked on asymmetric aldol reactions. He proposed the first set of “Proline catalyzed Direct Asymmetric Aldol reactions”.
Now, this seems a pretty complicated thing, so let us take a bit of time to feel what Aldol reactions are. These are simple condensation reactions in which water molecules are liberated as a by-product and other relevant products are formed. Prof. List is the first to report organic-based catalysts for these reactions.
The first to be studied by them in this regard is the reaction between acetone and nitro-benzaldehyde:
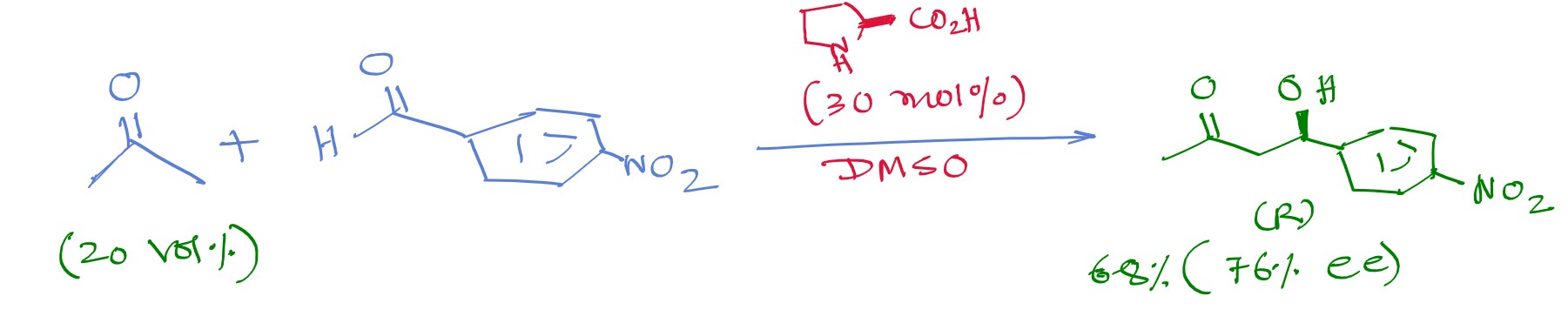 Proline catalyzed Asymmetric Aldol reaction.
These reactions were the first reported organic molecules to be used as catalysts in asymmetric reactions. Now, what is so good about organocatalysts like Proline? There are many reasons for this:
Proline catalyzed Asymmetric Aldol reaction.
These reactions were the first reported organic molecules to be used as catalysts in asymmetric reactions. Now, what is so good about organocatalysts like Proline? There are many reasons for this:
Now let us dive into Prof. MacMillan's work. He started to work on one of the most powerful reactions in the field of organic synthesis, the celebrated “Enantioselective Diels-Alder reactions”.
Diels-Alder reactions are one of the most widely used reactions in the field of organic chemistry. This reaction had itself bagged a Nobel Prize in Chemistry: such was its widespread usage. So, Prof. MacMillan’s further contribution to this reaction was like a cherry on the top.
Enantioselective reactions are ones in which only one compound is formed as a product selectively.
Prof. MacMillan gave a new dimension to this reaction and proposed using some particular organic catalysts to accelerate the reaction.
One of these catalysts was “Imidazolidinone” which was an organocatalyst that can get a particular product in the enantioselective Diels-Alder reaction.
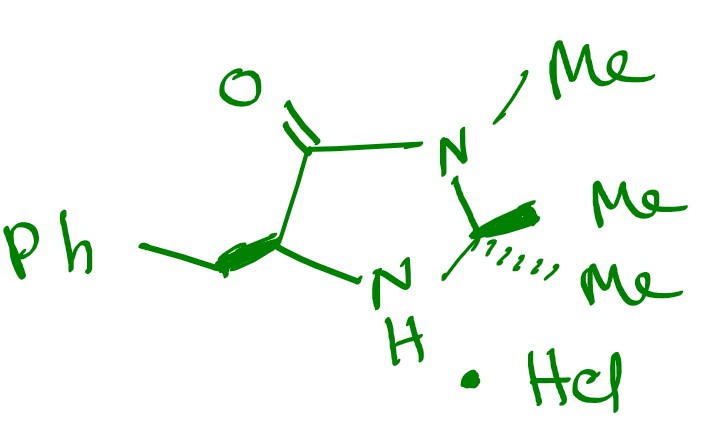 This is the Imidazolidinone catalyst developed by Prof. MacMillan for these Diels-Alder reactions.
How beautiful are these reactions!
This is the Imidazolidinone catalyst developed by Prof. MacMillan for these Diels-Alder reactions.
How beautiful are these reactions!
It is really intriguing to see and feel how both the legendary chemists started their journey on two different ships and ultimately landed upon the same island: That beautiful island is of "Asymmetric Organocatalysis”. This year's Nobel prize reflects how a pretty simple question can lead to significant conclusions and fancy results.
This extraordinary work has now gifted chemists a new magic wand to create molecules in a better manner without harming Mother Nature.
At last, we would bid a farewell to the wonderland of asymmetry for now with a famous quote from Jan Tschichold:
"Asymmetry is the Rhythmic Expression of Functional Design.”
Bibliography
Subhajit Chakraborthy is a 2nd-year BS-MS student at IISER K, INSPIRE fellow, NSEC gold medalist. He is an enthusiastic science geek who loves chemistry and aspires to do something in Natural Product Synthesis, Pericyclic chemistry and Photochemistry. Apart from studies, he loves to sing and entertain his friend circle with lovely songs.
signup with your email to get the latest articles instantly