
A Stranger Crashes the Party: Decoding the White Matter Wipeout
In the nervous system, myelin acts like insulation on electric wires - essential for fast, precise communication between neurons. But when viral infections invade the central nervous system (CNS), they can trigger immune attacks and cellular chaos that strip away this protective coating. Using single-cell analysis, uncovering how these “party crashers” disrupt myelin maintenance, reveals new paths to understand and potentially repair the damage in neuroinflammatory demyelinating diseases.
Do you know what gets on our nerves all the time?
Our friends, mostly! – Oligodendrocytes, the cells that form myelin in the CNS. They are our lifeline support system. How would you feel if they were stripped away from you by some strangers? Well, I can relate you with someone I know a bit closer – Neurons. You will be alone, drained of your refreshing communications and if you don’t find new promising friends sooner or later you become depressed until your last breath just like them.
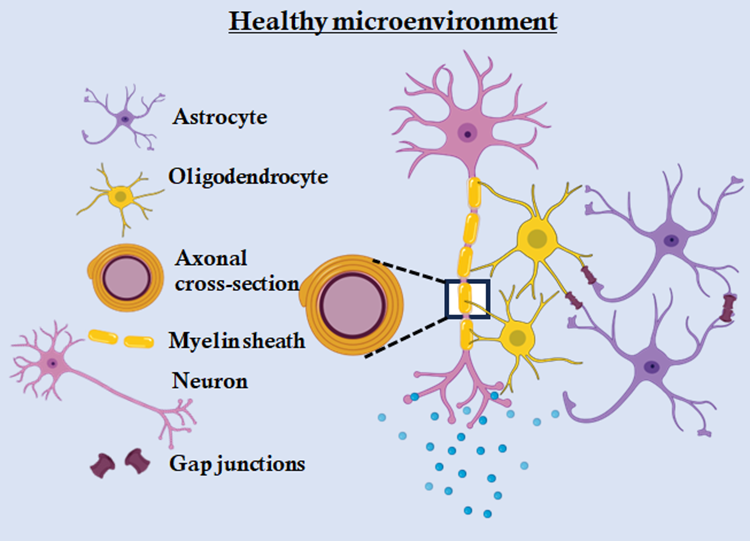
Demyelinating diseases of the CNS, such as multiple sclerosis (MS), are characterized by the destruction of myelin sheaths which are cytoplasmic extensions of oligodendrocytes around the axon of neurons and impaired remyelination (Fig 1). Chronic demyelination is often associated with neuroinflammation, glial dysfunction, and failure of oligodendrocyte progenitor cell maturation. While many studies focus on autoimmune or toxin-induced demyelination models, increasing evidence suggests that neurotropic viruses, including β-coronaviruses, the so called “strangers” can induce persistent CNS pathology characterized by chronic inflammation and impaired myelin repair [1-3].
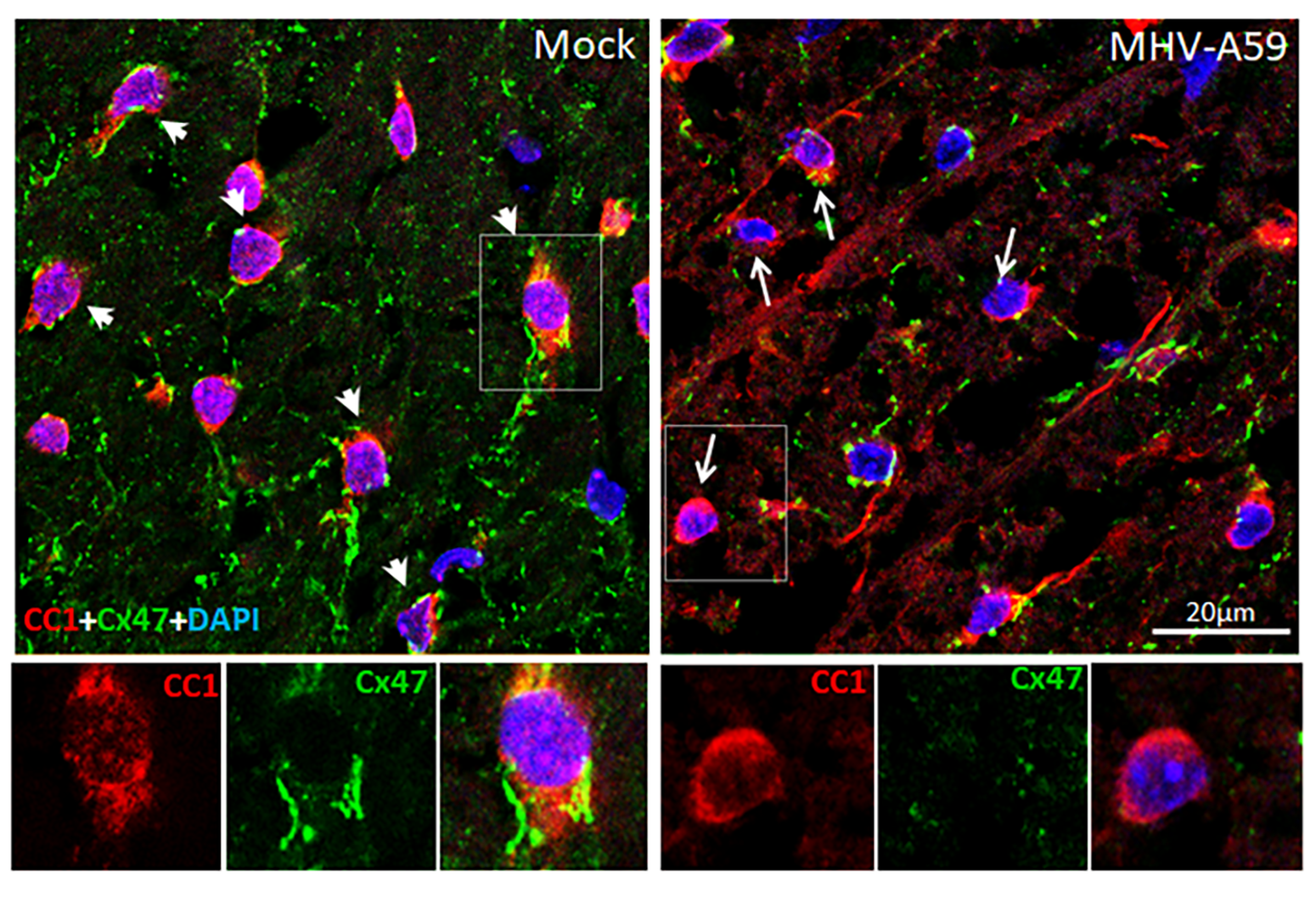
Mouse hepatitis virus strain A59 (MHV-A59), a neurotropic β-coronavirus, serves as a well-established model to study virus-induced demyelination. Intracranial infection with MHV-A59 leads to acute encephalomyelitis, which refers to the inflammation of both brain and spinal cord. This is followed by a chronic phase marked by demyelination and immune cell persistence in the spinal cord [4-6] (Fig 2). However, the mechanisms through which viral infection leads to chronic demyelination and the cellular and molecular landscape of the affected CNS remain incompletely understood. In our earlier study, in a targeted approach using immunostaining methods in spinal cord tissue sections collected from MHV-A59 infected and control mice, we observed significant variations in the proportion of different oligodendrocyte lineage cells upon infection, which correlated well with the extent of demyelination. Further we have reported differential regulation of cell junction molecules, such as connexin 47 (Cx47), in different oligodendrocyte lineage cell populations over time following infection [7].
Sharing is Caring: The Inner Network that keep the CNS in Balance
We all know how sharing with friends helps us stay connected and build strong relationships. Turns out, our brain cells feel the same way! They build tiny bridges - like secret passageways - to pass messages and share what they need to stay in sync. This constant exchange helps maintain balance and harmony in the CNS. Connexins constitute a large family of transmembrane proteins that are essential for the formation of gap junctions (GJs) - specialized intercellular channels that permit direct communication between the cytoplasm of neighbouring cells [8, 9]. Each gap junction channel is composed of two hemichannels (connexons), contributed by adjacent cells, with each connexon assembled from six connexin subunits. These channels facilitate the rapid and bidirectional exchange of ions, second messengers, metabolites, and small signalling molecules (typically <1 kDa), thereby enabling synchronized cellular responses. In the CNS, connexin-mediated GJs are critical for supporting neuroglial interactions, propagating calcium waves, and maintaining the ionic and metabolic balance required for brain homeostasis.
Disruptions in connexin function have been implicated in a range of neurological disorders, highlighting their importance in sustaining normal brain physiology and coordinated cellular behaviour. Our study revealed that Cx47 GJs are persistently lost in mature oligodendrocytes, not only in demyelinating lesions but also in surrounding normal appearing white matter areas during chronic demyelination (Fig 3). The loss of Cx47 in the oligodendrocytes is most likely triggered by an initial loss of its coupling partner, Cx43 in astrocytes, which occurs during acute viral infection. At later stages after viral clearance, astroglial Cx43 GJ expression re-emerge but mature oligodendrocytes mostly lack Cx47 GJ expression and thus fail to fully re-establish connections with the astrocytes. However, we observed appearance of Cx47 surface puncta in the oligodendrocyte precursor cells at this chronic demyelinating stage. This suggested of a plausible role of the Cx47 channels, modulated differently in the different oligodendrocyte lineage cells, in the demyelinating pathology induced by an initial viral infection. However, a key challenge remained to decipher how the individual cell states of the oligodendrocyte lineage cells interact with the other cell types in the tissue microenvironment upon an initial viral insult and contribute to the chronic pathological state.

It’s crowded: Let’s meet them in person
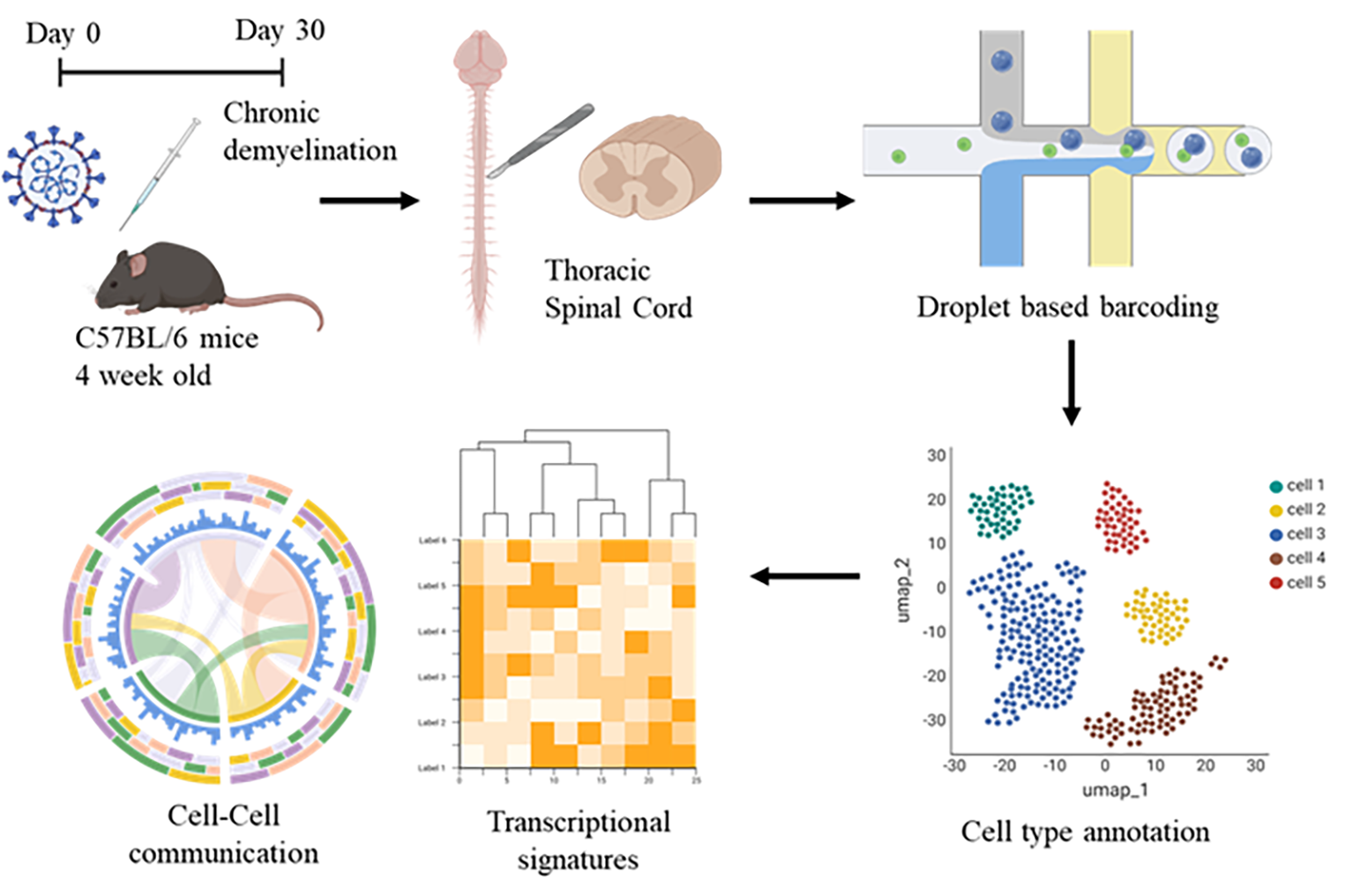
Imagine a stranger walks into your group of friends, who stirs things up. How would your friends react? Each one of them would have their own intrinsic reactions and opinions. It’s hard to figure out everyone’s reactions all at once. That’s a bit like what happens in our body - different cells respond differently to the same event. The complexity of the CNS comes from its incredible variety of cell types and their ever-changing interactions in health and disease. While traditional bulk RNA sequencing approaches have provided foundational insights into gene expression changes in demyelinating diseases, they obscure cell type–specific transcriptional signatures and fail to resolve rare or transient cell states critical to understanding disease progression. Single-nucleus RNA sequencing (snRNA-seq), which profiles RNA transcripts directly from isolated nuclei, offers several distinct advantages when applied to CNS tissues, especially in models of neurodegenerative or inflammatory disorders [10-12]. Particularly, snRNA-seq enables the profiling of gene expression at single-cell resolution while circumventing challenges associated with dissociating fragile CNS tissues, which can introduce stress-related artifacts or lead to selective loss of certain cell types having complex morphology during enzymatic digestion. This approach is principally advantageous in spinal cord tissue, where myelin-rich architecture and inflammatory changes can complicate live cell dissociation.
Thus, in our study we adopted this high-throughput method of snRNA-seq to classify thousands of nuclei into canonical CNS cell types—including neurons, astrocytes, oligodendrocyte lineage cells, microglia, and infiltrating immune cells—based on gene expression profiles (Fig 4). Sub-clustering further revealed disease-associated cell states, such as reactive astrocyte phenotypes (A1 vs. A2), reactive microglia, and disease associated oligodendrocytes (DOLs). Furthermore, snRNA-seq also captured immune cell infiltration with a population of T cells detected only in the infected spinal cord. This is critical in post-viral demyelination, where persistent peripheral immune cell infiltration is a hallmark of disease. Profiling the transcriptomes of these immune populations alongside CNS glia would help the identification of distinct cell–cell interaction pathways that may drive neuroinflammation and glial dysfunction leading to a demyelinating pathology. Dysregulated processes within specific cell types can be understood from pathway enrichment and gene regulatory network analyses. Our results show distinct gene expression signatures regulating myelination, inflammation, and neuronal function. Notably, oligodendrocyte lineage cells exhibit marked state transitions and altered interactions with other spinal cell types, potentially underlying persistent demyelination even after clearance of active viral infection. These findings establish the first single-cell atlas of the spinal cord in virus-induced chronic demyelination and provide critical insights into cell-specific and network-level mechanisms driving disease pathology.
From Discovery to Hope: Charting the Future of Demyelinating Disease Research
Understanding how a viral infection triggers chronic damage in the brain and spinal cord is a critical step toward unraveling the complexities of demyelinating diseases like multiple sclerosis. By combining advanced tools like single-nucleus RNA sequencing with detailed molecular studies, we are beginning to chart the intricate web of cellular interactions that go awry after a viral assault on the nervous system. Our research not only sheds light on how glial cells—especially oligodendrocytes—respond and adapt in this environment but also opens new avenues for exploring why repair mechanisms fail in chronic conditions. Moving forward, integrating these insights with cutting-edge technologies like spatial transcriptomics and lipidomics may reveal how persistent changes in cellular networks contribute to long-term neurological disorders, including those linked to emerging viral infections like SARS-CoV-2. The hope is that such research will pave the way for targeted therapies that can truly modify disease outcomes by restoring the delicate balance of cell communication within the CNS.
This work was made possible by significant contribution of the following: Sai Gayathri R, Soubhik Das, Supratim Ghosh, Archana KumariShaw, Subhajit Das Sarma, Jayasri Das Sarma, Arindam Maitra and Mahua Maulik
1 BRIC-National Institute of Biomedical Genomics, Kalyani 741251, West Bengal, India.
2 Indian Institute of Science Education and Research (IISER) – Kolkata, Mohanpur 741256, West Bengal, India.
This study is supported by funding from DBT/Wellcome Trust India Alliance Early Career Research Grant (Grant number IA/E/17/1/503659) and BRIC-NIBMG Intramural support to M.M.
References
- Bergmann, C.C., T.E. Lane, and S.A. Stohlman, Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol, 2006. 4(2): p. 121-32.
- Brola, W. and M. Wilski, Neurological consequences of COVID-19. Pharmacol Rep, 2022. 74(6): p. 1208-1222.
- Gasmi, A., et al., Neurological Involvements of SARS-CoV2 Infection. Mol Neurobiol, 2021. 58(3): p. 944-949.
- Das Sarma, J., et al., Mechanisms of primary axonal damage in a viral model of multiple sclerosis. J Neurosci, 2009. 29(33): p. 10272-80.
- Lane, T.E. and M.J. Buchmeier, Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol, 1997. 5(1): p. 9-14.
- Perlman, S. and A.A. Dandekar, Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol, 2005. 5(12): p. 917-27.
- Das, S., et al., Neurotropic Murine beta-Coronavirus Infection Causes Differential Expression of Connexin 47 in Oligodendrocyte Subpopulations Associated with Demyelination. Mol Neurobiol, 2025. 62(3): p. 3428-3445.
- Goodenough, D.A. and D.L. Paul, Gap junctions. Cold Spring Harb Perspect Biol, 2009. 1(1): p. a002576.
- Laird, D.W. and P.D. Lampe, Therapeutic strategies targeting connexins. Nat Rev Drug Discov, 2018. 17(12): p. 905-921.
- Lake, B.B., et al., Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol, 2018. 36(1): p. 70-80.
- Rosenberg, A.B., et al., Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science, 2018. 360(6385): p. 176-182.
- Russ, D.E., et al., A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat Commun, 2021. 12(1): p. 5722.
