
Cell Death Makes Living Easier
Progress in science depends on new techniques, new discoveries, and new ideas, probably in that order. — Sydney Brenner
Introduction
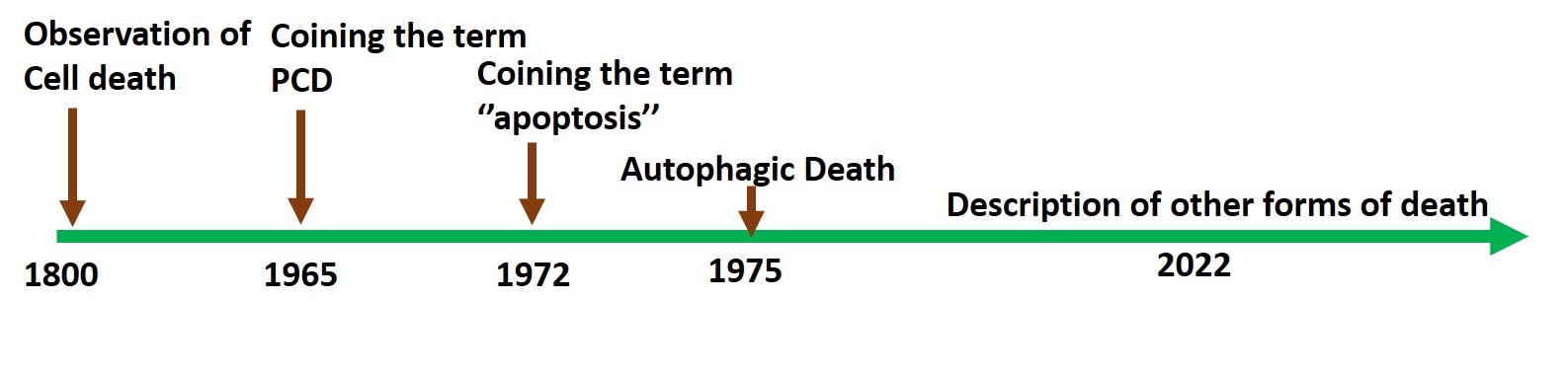
All living beings eventually die. Cells, as the fundamental units of life, became the primary focus of research to understand cell death. Over the past one and a half centuries, research on cell death has expanded, aided by groundbreaking advances in microscopy and biochemistry. The main focus has been on the possible origins and mechanisms of death when a cell dies either because it is aged or because it could not recover from injury (Fig 1). Because death paradoxically supports survival, questions about the process continue to fascinate biologists, chemists, and philosophers alike. This article will examine the intriguing questions and debates that dominate the field.

Scientists in the early and mid-19th century were interested in exploring how nature functions, because much was unknown. It was the time when Charles Darwin took the famous voyage of the Beagle and started working on the theory of evolution. His observations were published as a book titled “The Origin of Species” in 1859, and the theory of evolution by natural selection became the cornerstone of modern biology [1]. Around this time, Carl Vogt, a German scientist, who was studying the remarkable transformation of tadpoles into toads, put forth the theory that cells die to aid in the development of an organism from the embryo. Just over a century later, in the mid-1960s, a landmark paper was published by William Lockshin and Caroll Williams, describing cellular changes during insect metamorphosis, where cell death was necessary for shaping the organism. Importantly, they coined the term “Programmed Cell Death” (PCD), indicating the existence of a genetically programmed death process [2]. By then, immense advances in microscopy had allowed both macro and micro viewing, providing opportunities for great discoveries.
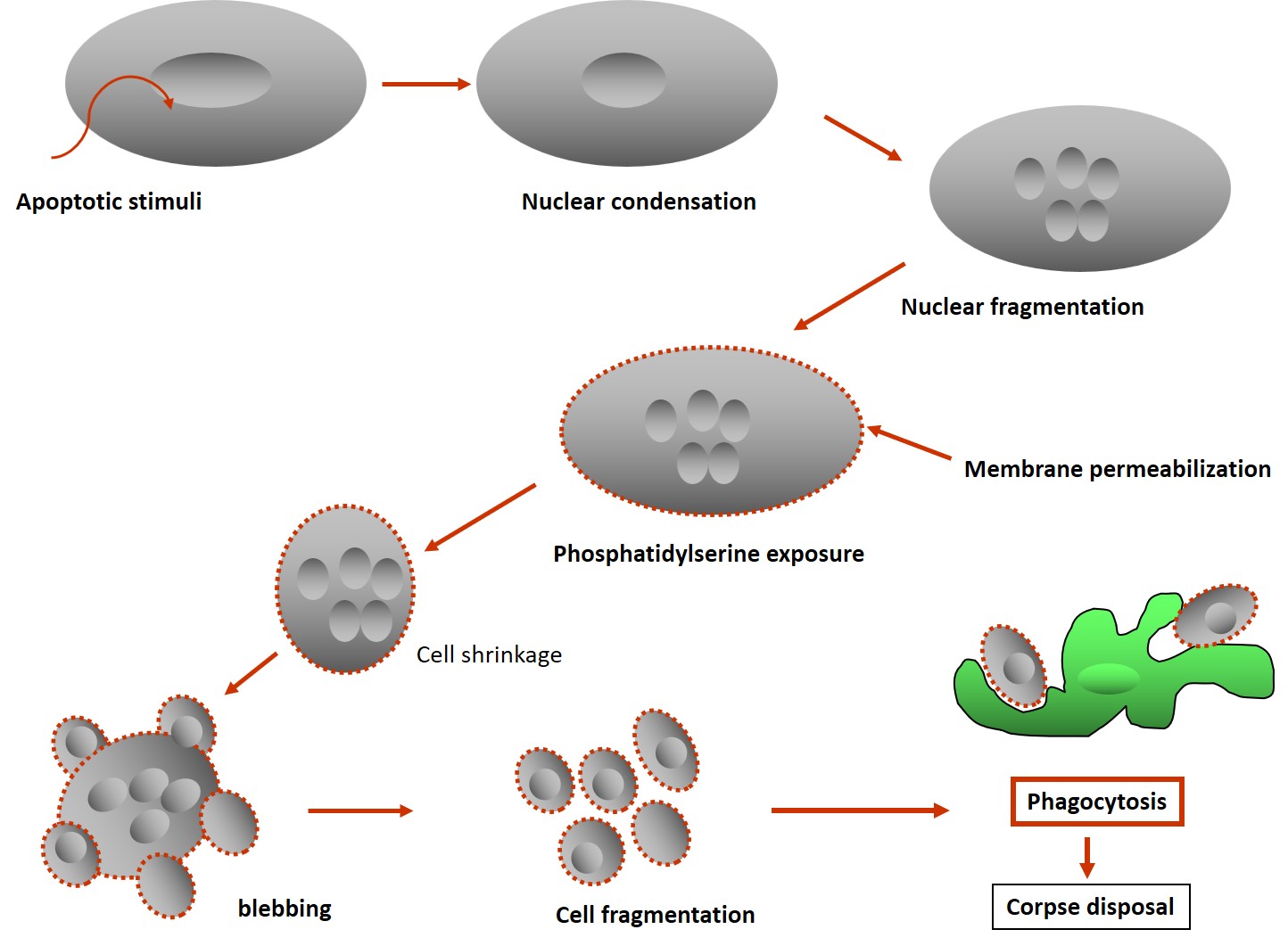
About seven years later, in 1972, three Australian scientists, Kerr, Wyllie, and Currie, published a landmark paper on cell death where they coined the term ‘apoptosis’ (Fig 2). It was inferred that complex biochemical processes brought about multiple morphological changes necessary for disposal of the dead cells or cellular corpse, by the macrophages. They defined a series of common cellular alterations, like cell shrinkage, nuclear fragmentation, membrane blebbing, and the formation of apoptotic bodies, that came to be recognized as the hallmarks of apoptotic death (Fig. 3). Apoptotic death is a subset of PCD that also includes other forms of death, with apoptosis being the most widely researched [3]. Between 1980–1990, genetic and molecular mechanisms related to apoptosis began to emerge, with the mapping of molecular pathways described between 1990 and 2000, and the therapeutic potential of the process and translational application trials started from 2000 onwards. Functionally, cell death supports cell survival by removing sick, damaged cells and cells with genetic errors to maintain homeostasis and is the major form of death during organismal development.
The symphony of apoptosis
The apoptotic process can be viewed as a symphony of signaling, like an orchestrated process with multiple players each having their specific roles, much like an intricate musical composition. The concert is required because when cells are eliminated from the body, the process needs to be precise to avoid damage to other cells. Therefore, the most important feature of the energy-dependent process of apoptosis is a clean form of death, in which cellular contents do not leak out during elimination. The apoptotic bodies that are generated, are membrane-bound forms picked up by phagocytes, the sentinels of the immune system, thereby preventing leakage of harmful lytic enzymes. Since the development of protein crystallography, the information on molecular interactions during the apoptotic process has led to a greater understanding of the molecular processes that regulate apoptosis. There are two main pathways of apoptotic death: the intrinsic pathway, mediated through the mitochondria, and the receptor-based pathway, where signals are received and transmitted by cell surface death receptors. For each of these pathways to induce apoptosis, specific enzymes called caspases are required to cleave substrates.
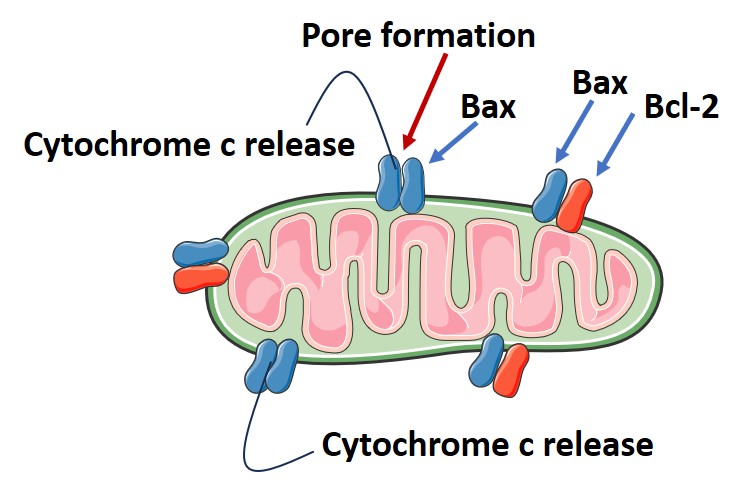
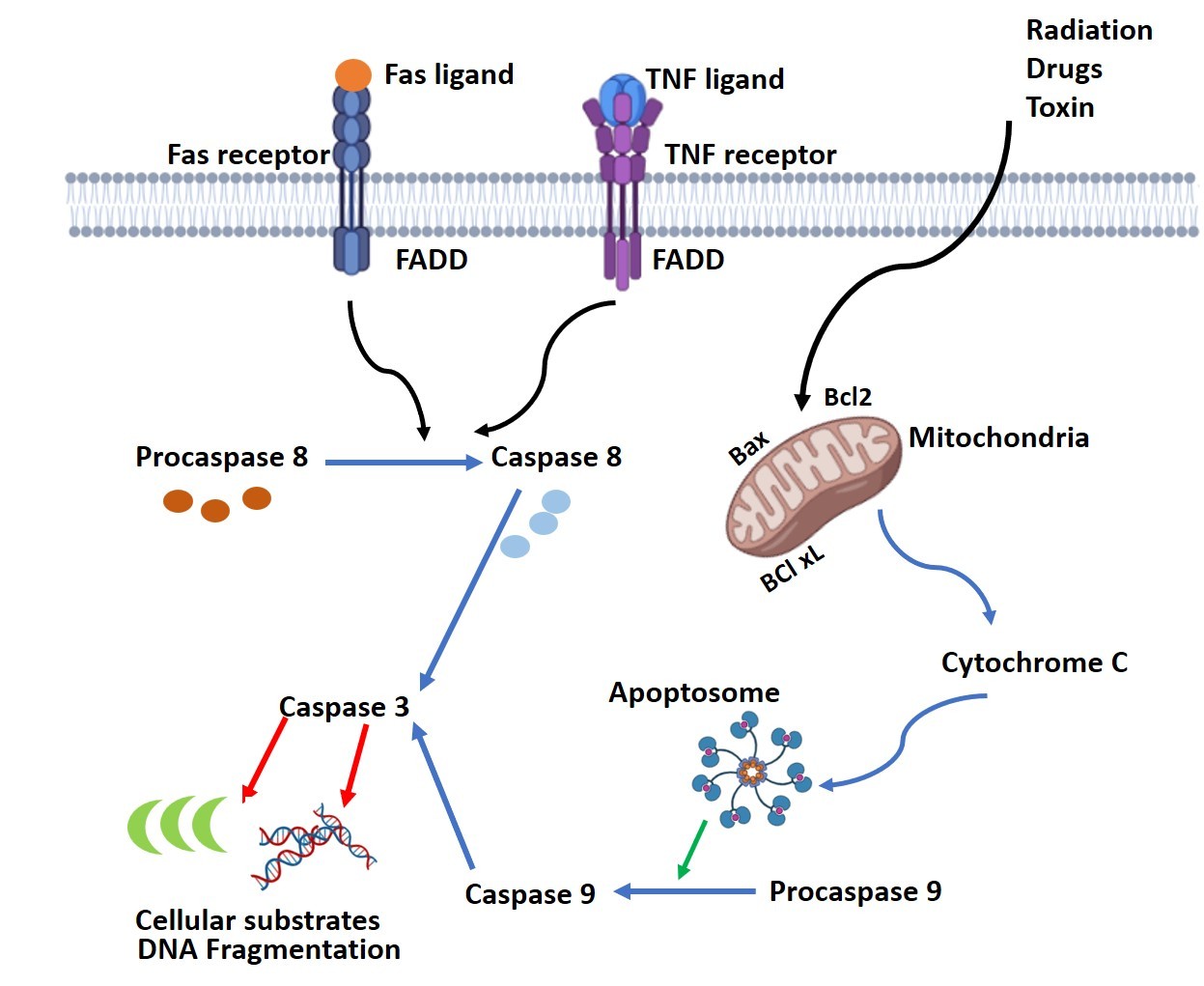
Caspases are a family of cysteine-aspartic acid proteases that are essential at various stages to complete the death process. They are produced as inactive pro-enzymes that are cleaved and activated as needed by the cellular machinery; otherwise, unnecessary cleavage could damage the cell. Functionally, two types of caspases exist: the initiator caspases activate another set of enzymes called effector caspases, responsible for digesting cellular components. The intrinsic pathway of apoptosis is mediated through the mitochondria via a complex interaction of pro- and anti-apoptotic molecules located on the mitochondrial membrane. When stress signals such as DNA damage, radiation, toxins, or oxidative stress are received, pro-apoptotic molecules like Bax and Bak and others form pores on the mitochondrial membrane. The formation of these pores, which is harmful to cellular processes, is normally prevented by the interaction of anti-apoptotic proteins like Bcl-2, BcL-xL, and MCL-1 with the pro-apoptotic proteins. As a result of pore formation, the release of cytochrome c, a component of the mitochondrial respiratory chain, occurs, initiating the dismantling process of the cell (Fig. 4). Cytochrome c release into the cytosol triggers the cytoplasmic protein Apaf-1 (apoptotic protease activating factor-1) to bind to it, forming the “apoptosome” complex. This complex recruits pro-caspase-9 and activates it to active caspase-9.
The receptor-mediated death process is triggered by the interaction of the death ligands with the cell surface death receptors like TNF-alpha and Fas receptor. This binding triggers FADD (Fas-associated death domain protein) to bind to the cytoplasmic part of the receptor and recruits procaspase-8 to form a structure named DISC (death-inducing signaling complex). Here, the activation of procaspase-8 is completed. Both caspase-8 and -9 are initiator caspases and activate the executioner caspases-3 or -6, or -7 to cleave cellular components like the cytoskeletal proteins, DNA repair enzymes, nuclear lamins, and DNA (Fig 5). As a consequence of these enzyme activities, the cell shrinks and breaks into small fragments called the apoptotic bodies, which are engulfed by phagocytes, the sentinels of the immune system, ensuring clean removal of cellular debris as referred to earlier. Apart from the above two pathways using caspases, there is a caspase-independent death pathway where cells undergo apoptosis using a mitochondrial protein, the Apoptosis-Inducing Factor (AIF), that travels from the mitochondria to the nucleus to degrade DNA after the cell has received a death signal [4].
Elegant models for apoptosis research
For any research program in biology, suitable cell systems or model organisms are necessary. These can be selected based on the research question, ease of technical manipulation, and similarity to the human system. Certain organismal models have been favorites for apoptosis research. A transparent worm, Caenorhabditis elegans, consisting of 1091 cells, where 131 cells die at a specific time during development by apoptosis, has been used in many studies [5]. Three collaborators, Sydney Brenner, Robert Horvitz, and John E. Sulston, used C. elegans as their model to study the apoptotic process. Their discoveries on programmed cell death were awarded the Nobel Prize in Physiology or Medicine in 2002. Their research revealed the involvement of four primary genes, EGL-1, CED9, CED4 and CED3, for apoptosis (Fig. 6). Genes with similar functions were identified in humans. Apart from C. elegans, other organisms used for apoptosis research include Drosophila melanogaster [6], Mus musculus, and Danio rerio [7]. Apoptotic pathways in Mus musculus are highly conserved with humans, making it a suitable model for studying mammalian apoptosis. Danio rerio is ideal for studying developmental apoptosis.
Why did selection choose apoptosis?
As developments in molecular and cell biology progressed, more and more new questions arose. Self-induced death as apoptosis was viewed as an evolutionary puzzle because selection is expected to favour the development of processes that help the individual to avoid death and propagate. Then how was the selection of the death process possible? At this juncture, ideas enforced the belief that the origin of PCD coincided with the emergence of multicellular organisms on Earth. The first multicellular organisms appeared around one billion years ago. Were they the first organisms whose cells were capable of self-destruction for the benefit of others? It was perceived that due to a close network of cells, some members could undergo suicide to protect others in their immediate niche. It was believed that selective pressure acted on the multicellular body to evolve cell death as a mechanism of survival because it is the evolutionary ‘unit of selection.’ Because biological systems are hierarchically organized, selection could operate at different levels, leading to cell death occurring at multiple levels without affecting the whole. Therefore, self-destruction through PCD could be seen as an extreme form of cooperation that is costly to the lower level but beneficial for the higher level, that is, at the level of the organism [8].

Is apoptotic death possible in unicellular organisms?
Apoptosis in unicellular organisms is a fascinating idea because, being single-celled, they do not need to act altruistically toward others. Or do they? In unicellular organisms, “cell suicide” or apoptosis through PCD may not be beneficial because, evolutionarily, death at the individual cell level could be considered a failure. Therefore, any gene that codes for death would be negatively selected. The notion that apoptosis evolved for the benefit of multicellular organisms started to shift around the mid-1990s when cell death was observed in unicellular organisms across various phyla. These included prokaryotes, phytoplankton, autotrophic and heterotrophic flagellates, yeasts, slime moulds, and ciliates. Studies investigating caspase homologues (metacaspase, orthocaspase, and paracaspase) containing the p20 domain—which includes the catalytic dyad formed by histidine and cysteine—revealed the presence of structural homologues widely spread across archaea, bacteria, and eukaryotes. This indicated the presence of some form of apoptotic process in these organisms.
However, para-caspases have not yet been shown to induce PCD functions. The question was, can death be a better strategy than survival for unicellular life forms? Who would benefit? The pursuit of answers regarding how cell death was selected in single-celled organisms and how it might benefit them led to multiple theories. It was suggested that, since unicellular organisms spend most of their lives in multicellular communities, they have developed communication methods through which they receive, detect, interpret, and respond to signals from others (Fig. 7). This behavior mimics multicellular functions. Examples include quorum sensing, where single-celled organisms respond to population density via signaling molecules; responses to chemical cues; electrical signaling; light-based communication; or horizontal gene transfer, as observed in bacteria.
Research suggests that cell death evolved in single-celled organisms due to their complex existence. Therefore, it is possible that cell fate, in terms of life and death, may also be influenced by an evolutionary scenario involving social control of death at the colony level. Unicellular populations that are mostly clonal share close genetic similarity. Also they stay in close communities, either as a community of the same species or a combination of multiple species (Fig. 7). Consequently, altruism expressed by individual members would benefit other members of the cell pool, but it would be costly to the altruistic individual. Possibly, mechanisms of kin selection are employed, where individuals support relatives who share a significant portion of their genes, thereby enhancing their inclusive fitness. It appears that bacteria possess mechanisms that may influence internal death, mortality from an arms race, competitiveness, cooperation, selfishness, and altruism.
What benefit is it to the unicellular organisms of expressing apoptosis? Given that they live in close associations, they use apoptosis to discard unfit cells to help the colony to be healthy. Restriction in numbers is an important issue in any niche so as to not to create a stressful situation through overcrowding. Apoptosis is the preferred way because it happens without the release of harmful agents in a colony, therefore, a clean removal benefits others. This is also true for infectious agents, as they have to restrict their numbers so as not to kill the host. In this context, it is pertinent to mention that the infectious agents have developed an arsenal of mechanisms that have the potential to thwart the host’s protective responses.
The same fundamental elements of the cell death program are activated in various cell types, whether they respond to developmental signals, immune signals, oxidative stress, injuries, or infections. The numerous components of cell death machinery are evolutionarily conserved from worms to humans; however, a lot needs to be researched for similarities and peculiarities of the cell death pathways in single celled life to understand the thread that exists between the uni and multicellular life [9,10]. Initial selection of the functional domains of molecular effectors that control death, aging, and adaptation might have favored their original selection, and this is true for both unicellular and multicellular organisms. Some of the molecules associated with cell death may have been associated with survival pathways that were later adapted for the death signalling. Cell death in unicellular organisms remains an attractive area for further research to understand an important component of the biology of survival.
References
- Berry A, Browne J. PNAS. U S A. 26:119: 2022. doi: 10.1073/pnas.2122144119.
- Lockshin RA, Williams CM. J Insect Physiol. 11:123-33. 1965 doi: 10.1016/0022-1910(65)90099-5.
- Kerr JF, Wyllie AH, Currie AR. Br J Cancer. 26:239-57. 1972 doi: 10.1038/bjc.1972.33.
- Yuan J, Ofengeim D. Nat Rev Mol Cell Biol. 25:379-395. 2024. doi: 10.1038/s41580-023-00689-6.
- Zuryn S. Semin Cell Dev Biol. 15;154(Pt A):1-3. 2024. doi: 10.1016/j.semcdb.2023.07.006.
- Zhou L. Adv Exp Med Biol. 1167:105-112. 2019. doi: 10.1007/978-3-030-23629-8_6
- Costa B, Estrada MF, Mendes RV, Fior R. Cells. 2020 9:293. doi: 10.3390/cells9020293.
- Ameisen JC. Cell Death Differ. 9:367-93. 2002. doi: 10.1038/sj.cdd.4400950.
- Kulkarni M, Hardwick JM. Annu Rev Genet. 57:435-459. 2023 doi: 10.1146/annurev-genet-033123-095833.
- Van Dyken JD, Zee PC. Am Nat. 204:468-481. doi: 10.1086/732199.
