How ‘cool’ is Absolute Zero?
Review
Simli Mishra
Absolute zero is the lowest temperature theoretically possible which is also practically impossible to achieve. It is the temperature which the Universe is tending to in its theoreised eventual heat death. A number of interesting phenomena occur near this temperature which have intrigued scientists for a century, and it also holds the key to future engineering.
Tweet
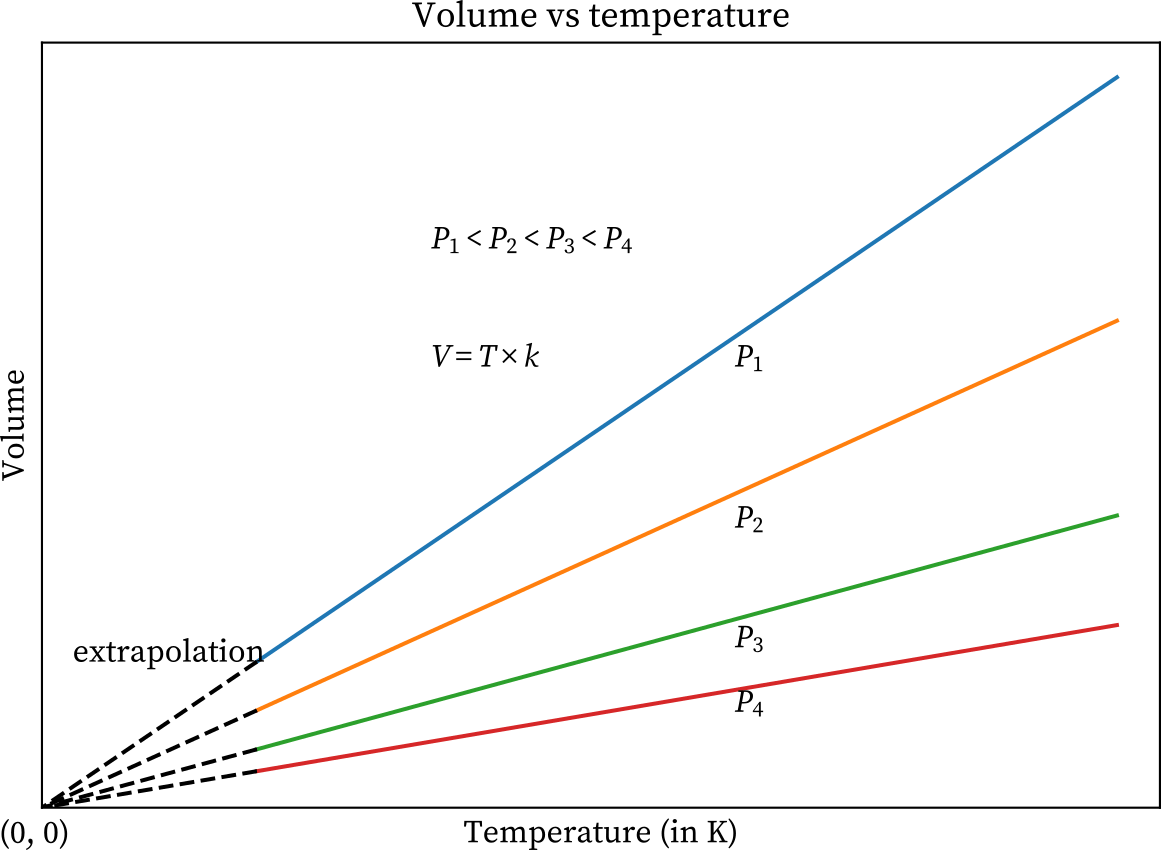
‘Absolute zero’ is where there is no motion of molecules. An ideal gas (a hypothetical gas whose molecules occupy negligible space, have no interactions and obey the gas laws) attains zero volume if they are cooled to -273.15 ℃ or 0 K at constant pressure. However, reaching this temperature would require an infinite amount of energy. “Quantum physics states that it is impossible for a particle to be fully at rest in a specific location,” says Alessandro Toschi, Associate Professor at TU Wien. “Heisenberg's uncertainty principle tells us that position and momentum cannot be ascertained with total precision. Therefore, a particle's position and momentum can still change at absolute zero.” 1
 The scientific journey of cooling and liquefaction of gas in laboratories began long back to understand the physical properties of different materials and different kinds of interactions happening in a system. In 1845, Michael Faraday became one of the first scientists to liquefy gases. Following his work, many gases, including oxygen, nitrogen, and hydrogen, were also liquified. As Kamerlingh Onnes finally liquified the last remaining gas, helium at 4 Kelvin (-269℃) in 1908, it revolutionized experimental research and unraveled hundreds of interesting phenomena, happening at that new extreme.
The scientific journey of cooling and liquefaction of gas in laboratories began long back to understand the physical properties of different materials and different kinds of interactions happening in a system. In 1845, Michael Faraday became one of the first scientists to liquefy gases. Following his work, many gases, including oxygen, nitrogen, and hydrogen, were also liquified. As Kamerlingh Onnes finally liquified the last remaining gas, helium at 4 Kelvin (-269℃) in 1908, it revolutionized experimental research and unraveled hundreds of interesting phenomena, happening at that new extreme.
The use of liquid hydrogen as a fuel for rockets or liquid helium in orbiting infra-red telescopes are just a few examples from an entire pile of applications of cooled and liquefied gases. Perhaps the greatest application in modern times is in the study of superconductivity, where resistance that a material offers to the flow of current, drops down to zero. This means current can flow through superconductors without any loss of power at all.
At a very low temperature liquid helium behaves as a ‘superfluid’, that is, it flows on a surface without any friction. If you put some of it in a circular channel and get it flowing, it will do so forever, not slowed down by friction.
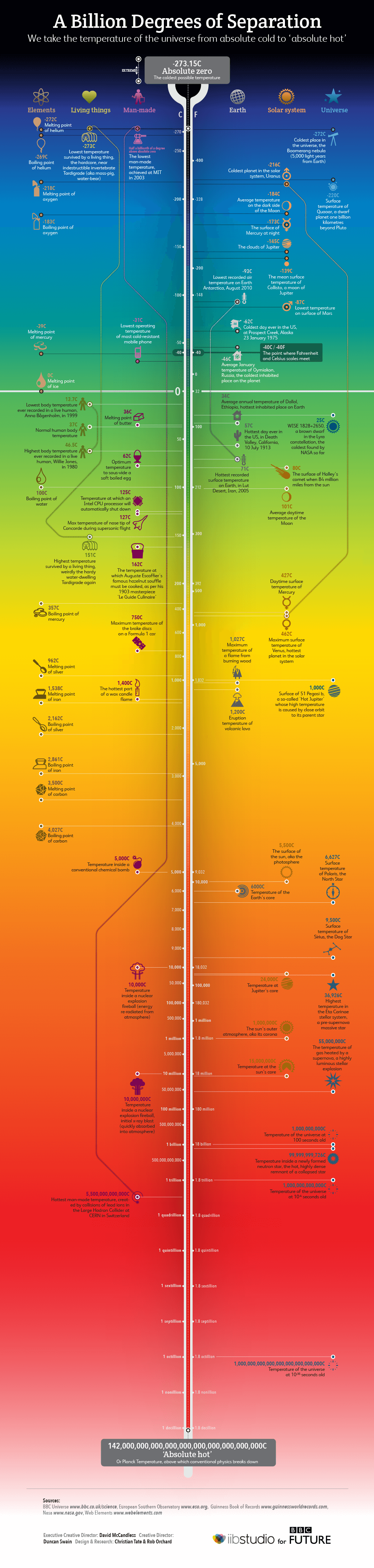
To give a perspective of how low that temperature is: average body temp is 310 K (or 37℃) and at 33℃ you will suffer from amnesia. If your body temperature drops to 294K (or 21℃), you might die of hypothermia. The coldest habitable place on earth in Oymyakon, Siberia, experiences a temperature of around -60℃ in the winter months2. The minimum temperature measured on earth, at the ice-covered southern continent is -89℃. It was measured at Soviet Vostok station, Antarctica on 21st July 1983 3. In the solar system, Uranus holds the title for the lowest recorded temperature of -224℃. Boomerang nebula, 5000 light-years away from earth is known to be the coldest place in nature with a measured temperature of just -272.1 ℃ or 1K, that is just one degree above the absolute zero 4.
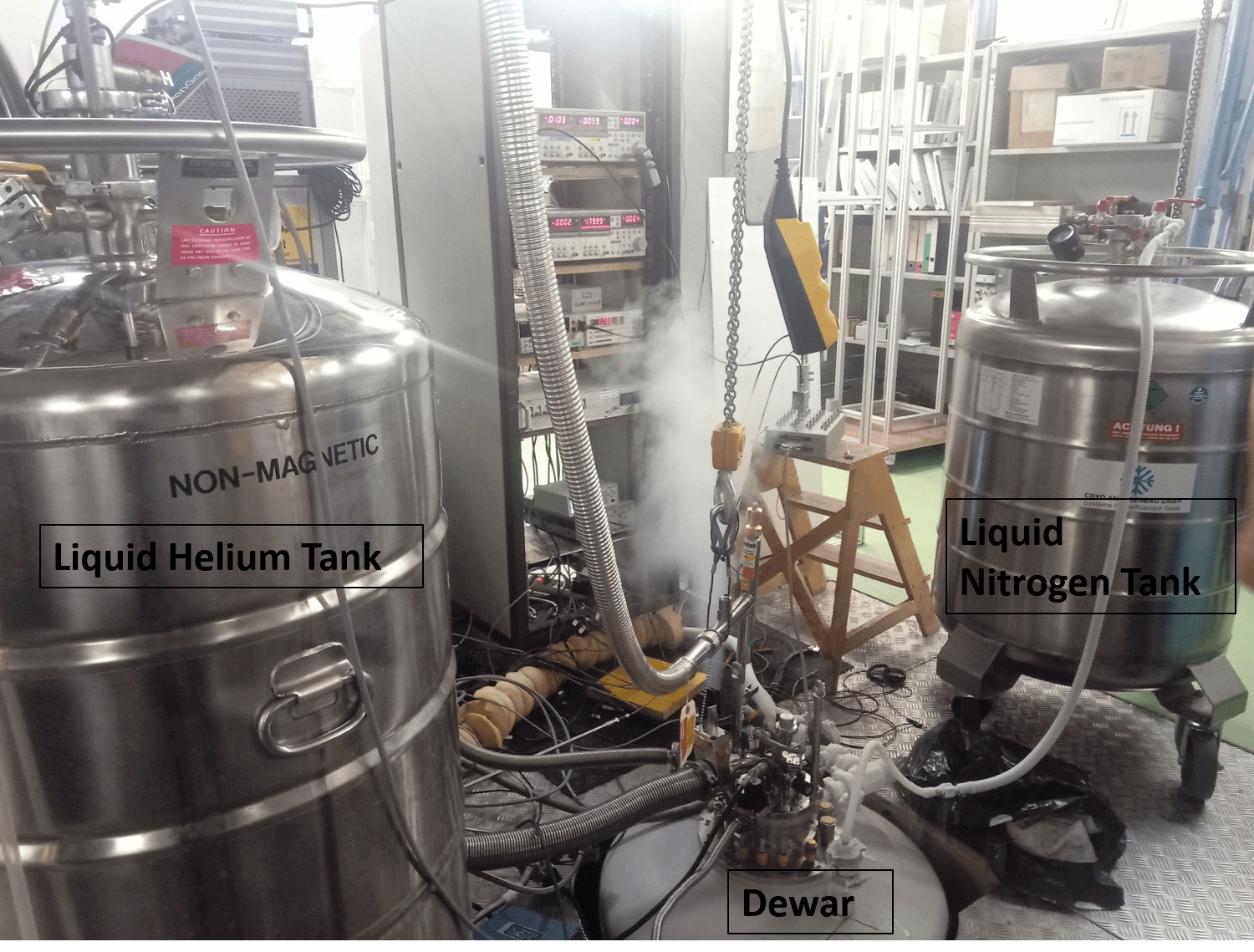
I had the pleasure of observing this phenomenon myself. We used a sophisticated assembly of huge containers, pipes, vacuums, electronics inside a huge container called a “Dewar”. It started from something around 250 K and slowly went down to 77K. After a couple of people went and changed a few settings and added a liquid into the huge ‘Dewar’, the temperature dropped again to 4K (Liquid Helium), only 4 degrees above absolute zero. As the minutes flew by, the temperature further dropped to 1.5K, and finally settled at an astounding 0.027K.
A temperature much lower than what exists in nature was achieved in a complicated laboratory equipment right beside me. I stood there watching, awestruck - in a medium-sized laboratory in a small town of Europe.
It is amusing how technology has taken us so close to the absolute zero, to an asymptotic limit, which is theoretically impossible to achieve. The lowest temperature measured in a laboratory was recorded to be 0.00036K at the National Institute of Standards and Technology (NIST) in Boulder, Colorado.5
What is even more astonishing is that such a low temperature can be attained by using an elementary principle, the same principle that causes us to sweat more on a hot day, that evaporation causes cooling. Except in this case we use a mixture of two types of helium in their liquid state, instead of sweat.6
A simple analogy to understand this process would be our habit of blowing air into hot water to cool it down, before we can drink it. Instead of air and water, this process uses a lighter (concentrated with 3He) and a heavier (dilute with 3He) form of a 3He and 4He mixture. And, instead of blowing air, we use a vacuum. The evaporation of 3He from concentrated to dilute form causes the temperature to fall down drastically.
For someone who is looking forward to performing experiments at such low temperatures, these dewars, refrigerators, and cryostats hold the key to delightful scientific surprises. It is indeed pretty cool to find answers to what happens in the extreme temperatures near absolute zero.
Bibliography
Simli Mishra, just finished final year BS-MS with a major in Physics, from IISER Kolkata. Her research interest lies in the field of experimental condensed matter physics, with particular focus on exploring correlated electron systems at very low temperatures. She will be joining Max Planck Institute in Dresden for her PhD.
signup with your email to get the latest articles instantly