Mauve : A happy accident
Review
Shrestha Chowdhury
 Photo credit: https://www.ancientearthpigments.com/product/purple-violet/
Photo credit: https://www.ancientearthpigments.com/product/purple-violet/
Imagine a world where you have to wear the same colour every single day. How gloomy that would be! The modern world is saturated with colours. But there was a time when making colours was a luxurious enterprise. In this article is presented the fascinating and riveting tale of an artificial dye that changed one man’s fate and revolutionised the world we live in.
Tweet
The English language is rich with colorful phrases. Perhaps you are familiar with the idiom “blue blood”. It refers to a person having a royal lineage. Similarly, the color purple also has a long-standing connection with aristocracy and luxury. Purple was worn exclusively by the kings in antiquity. It was a symbol of high status and power. In modern days, when people use the term “born to the purple”, they refer to a person of privileged birth.
In ancient times, dyes were not produced artificially. Instead, people arduously extracted pigments from different parts of plants and animals. Moreover, the colours faded over time. As a consequence, colored robes were very expensive. The purple of the ancient times, called Tyrian purple, was obtained from the secretion of whelk shells(1). Archaeologists have discovered heaps of whelk shells on the sea beaches of Tyre and Sidon. Those cities were involved in the production and trade of the dye. It is said that around nine thousand whelk shells were killed just to make one gram of this fascinating purple dye. Gorgeous purple colored robes were not only adorned by Byzantine kings but also by Roman senators. The dye was so much in demand that whelk shells were almost about to be extinct once!
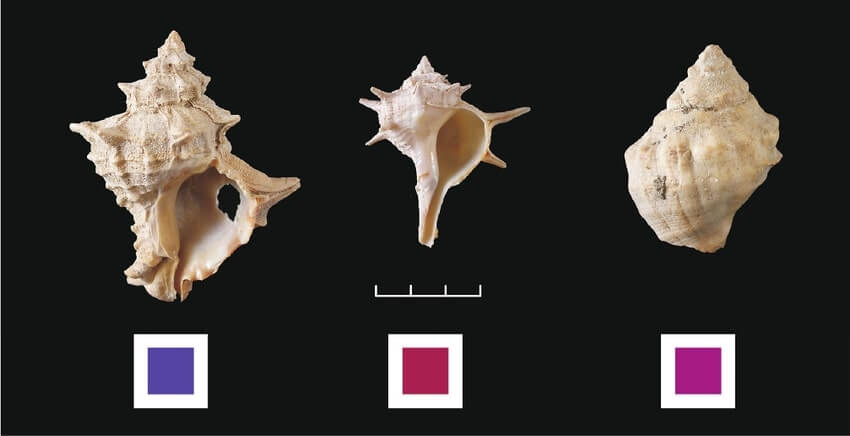
Fig 1.Various species of whelk shells were used to prepare different shades of purple.
Photo credit: The Tyrian purple,” a royal dye”, by Rena Veropoulidou
During the era of imperialism, many Europeans settled across various parts of the world. Consequently, they got affected by tropical diseases like malaria. The only treatment of the disease was quinine - a drug obtained from the bark of the cinchona plant. The plant is indigenous to South America. With its increasing demand for production and exportation, the cost of the bark scaled up. The plant also faced the risk of being endangered. An alternative had to be found. Chemists eventually took up the challenge to make synthetic quinine.
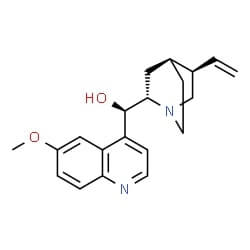
Fig 2.Chemical structure of quinine molecule
Image credit: ChemSpider
A bright young 18 year old student named William Perkin made the endeavour after it in his own home laboratory. It was the Victorian age, and the industrial revolution was at its peak. Naturally, coal tar was abundant and cheap. Perkin's teacher at Royal College of Chemistry, Dr. August Wilhelm von Hofman, was convinced that quinine could be prepared from coal tar.
 Fig 3. Sir William Henry Perkin
Fig 3. Sir William Henry Perkin
Sir William Henry Perkin (1838-1907) is a painting by Granger which was uploaded on August 24th, 2016.
Perkin attempted several trials but none of them were successful. In one of his attempts in 1856, he ended up with a black substance which upon dissolution in ethanol produced brilliant purple color. He dipped a piece of silk into the solution and much to his amusement found the color retained. A happy accident had occurred. He knew the fact that purple was rare and expensive to prepare in the dye industry. Being a man of genius insight, he at once sent a piece of the dyed cloth to an eminent dye company in Scotland.
Gaining the consent to perform mass production of the dye, he ventured to start up a business of his own. He took a patent for his newly made compound, which he named mauveine: the French name of the mallow flower. Over 15 years, Sir William Perkin made a colossal fortune and earned fame. In 1859 mauve, Perkin’s violet was in vogue in Victorian society. In fact, even bridal dresses got dyed in purple. Purple was also used in British postal stamps. The 1890s was called the mauve decade(2).
So far, so good; but where did Perkin go wrong in making quinine?
People in those times were not as well equipped with spectroscopy and other techniques as we are now. They could only make assumptions of the chemical structure instead of actually detecting them. Perkin made wrong assumptions right from the very beginning. He knew that the molecular formula of quinine is C2oH24N2O2. He also knew that allyl toluidine had a chemical formula of C10H13N. He thought that combining two molecules of allyl toluidine in the presence of potassium dichromate might result in oxidation to form quinine. Although the reaction seems easy going according to chemical balance, it does not, in reality. take place in that. Knowing the correct steps for synthesis depends greatly upon knowing the accurate chemical structures of parent compounds. Thus, Perkin ended up with a completely new spectacular-looking molecule called mauveine instead of quinine.

Fig 5. Hypothetical balanced equation showing formation of quinine from p toluidine
Perkin established that a similar purple dye could also be obtained from the oxidation of xylidines and other anilines instead of toluidine(3). He could eventually figure out that mauveine was derived from a parent compound having the molecular formula of C27H24N4. He also admitted that impure aniline gave a better shade of the colour compared to pure aniline. He further mentioned that mauveine was a mixture of pseudo mauveine and a tri methyl derivative of p toluidine and aniline. Present NMR studies reveal the part of the component responsible for the mauveine colour.
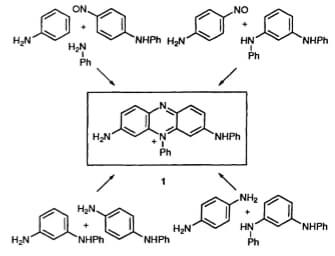
Synthesis of pseudo mauveine (the enclosed molecule)(4).
Mauveine is the first artificial dye made up of aniline. Coal tar dyes eventually came to be known as aniline dyes. Perkin single-handedly showed chemistry as a possible way of successful entrepreneurship. At face value, he did invent a new color; but he indisputably also opened the door to immense prospects in large-scale organic chemistry synthesis.
Bibliography
Shrestha is currently working as a senior research fellow in Department of Chemistry in IISER Kolkata. She has been a member of the editorial team of Cogito137. She is an avid reader, loves plenty of solitude and possesses fair inclination towards creative projects.
signup with your email to get the latest articles instantly